Chinese Medicine Research on Lung Cancer
Introduction to Lung Cancer and Its Treatments

Lung cancer is the leading cause of cancer deaths in the world, causing up to million deaths annually. 1 Less than half of lung cancer patients live more than 1 year and only 15% of them live for 5 or more years. The overall 10-year survival rate for lung cancer patients is only 7%. 2 There are two major types of lung cancer , namely small cell and non-small cell lung cancer, which describes the size and appearance of the malignant cells as seen under the microscope. Non-small cell lung cancer is the more common type, account ing for about 80% of all primary lung cancers. 3 Significant progress has been made over the past decade in the treatment of advanced non-small cell lung cancer (NSCLC). Treatment options are determined by the type and the stage of cancer and can include surgery, radiotherapy, chemotherapy or target biological therapy. While combination chemotherapy has been developed to improve the outcome of advanced NSCLC, Chinese medicine and herbal products are commonly used by patients who also seek conventional therapies for advanced NSCLC. Pharmacological studies have indicated that certain oriental mushroom extracts such as polysaccharopeptide (PSP) obtained from Coriolus versicolor has anticancer and immunomodulatory properties.
Chinese Medicine Research
Coriolus vesicolor is also known as Yunzhi or "cloud mushroom" in Chinese. This name is given because of the interesting wave patterns found on the mushroom caps. TCM practitioners of centuries ago understood that this mushroom has special health properties, which are good for maintaining general health and delaying the normal aging process if taken appropriately. Yunzhi first caught the attention of researchers in China in 1984 when noted fungi expert, Professor Yang Qin Yiao, successfully made a technological breakthrough in the structural elucidation of polysaccharopeptide (PSP), which is the active ingredient of Yunzhi responsible for its health benefits. PSP has been shown to manifest immunomodulatory and anticancer properties in both pre-clinical experiments and clinical trials. It has been shown to reduce the side effects of radiotherapy and chemotherapy and has been used as an adjunct medical modality to conventional cancer treatment. Experiments suggest that PSP can boost the immune system and alleviate the symptoms of chemotherapy. 11Pre-clinical Mechanistic Studies:
In vitro , PSP is effective for activating T lymphocytes, B lymphocytes, monocytes, as well as promoting the proliferation and production of antibodies and various cytokines such as interleukin-2 and interleukin-6. 4 Numerous in vivo studies have also revealed that PSP is capable of restoring certain depressed immunological responsiveness caused by tumor progression, chemotherapy and radiation therapy. 5, 6 Several studies reported that PSP possesses selective anti-cancer activity against certain cancer cells. PSP dose-dependently and time-dependently suppresses proliferation of human cancer cell lines. Xu showed that PSP markedly inhibited the growth of several human cancer cell lines including lung (SPC) cancer cell line s. 7 Similar findings also indicate that PSP can act selectively in HL-60 leukemic cells by arresting the cell in the G-phase of the cell cycle and including apoptosis but not affecting normal lymphocytes . 8 In vivo anti-tumor activity of PSP has also been extensively studied. Significant tumor size reduction was shown after prolonged administration of PSP in mice inoculated with lung adenocarcinoma (Lewis lung cancer) .Clinical Efficacy Studies:
Since there is lack of clinical trials on the efficacy of PSP in adjunctive lung cancer treatment, a clinical study was conducted by Dr. Kenneth Tsang at the University of Hong Kong's School of Medicine in 1999, on the PSP treatment of patients with advanced non-small cell lung cancer. 10 This study was a phase II double-blind placebo - controlled randomized clinical trial in 68 patients with advanced NSCLC who were equally recruited into PSP treatment and placebo group respectively. Patient enrollment commenced from 1999 to 2001 with the inclusion criteria of having a Karnofsky performance scale bigger than 60, life expectancy longer than 12 weeks and TNM stage III or IV ( II ). Patients who had radiotherapy or chemotherapy were also permitted to take part if they completed treatment at least four weeks prior to study. Eligible patients were randomized by taking either three capsules of PSP (340mg each) or an identical placebo (350mg crystallized sucrose each) three times a day for four weeks. Clinical and laboratory evaluation of patients was performed at the beginning and after the four-week treatment. After the four-week treatment, there was a significant increase in blood leckocyte and neutrophil levels and body fat compared with pre and post treatment of PSP. Serum IgG and IgM were significantly improved in the PSP treated group compared to the placebo group after four weeks In addition, there were less PSP treated patients who withdrew from the study due to disease progression. Therefore, this study suggests that PSP treatment may be of some benefit in patients with NSCLC. (See table 1)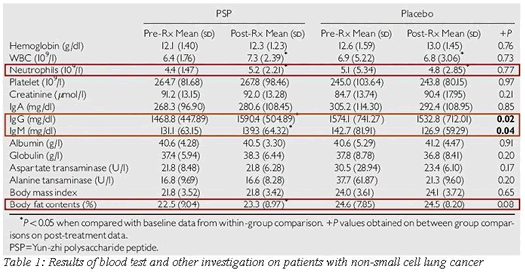
Conclusion
A substantial number of preclinical and clinical studies continue to suggest PSP administration may be a useful adjunct to conventional cancer therapy. While PSP is commonly used by patients who access conventional cancer care, further preclinical studies are necessary to establish its mechanisms of anti-cancer and immunomodulatory action and clinical trials are needed to prove the mechanistic effects that have been observed in vitro and in animal studies. For cancer patients who view conventional medicine with ambivalence, practitioners can foster a more open and communicative relationship by demonstrating an objective understanding of both alternative and conventional approaches. Using alternative and complementary medicine treatments may be able to improve the quality of life for those suffering from terminal diseases such as lung cancer.References
- Parkin, D.M. et al. Global cancer statistics in the year 2000. Lancet Oncol. 2, 533-543 (2001).
- Hurria, A. et al. Management of lung cancer in older adults. CA-Cancer J. Clin. 53, 325-341 (2003).
- Lam, W.K. et al. Chemotherapy for advanced (stage III B and stage IV ) non-small cell lung cancer: the Hong Kong perspective. Respirology3, 145-149 (1998).
- Li, X.Y. Advances in immunological studies in PSP. in Advanced research in PSP (ed. Yang, Q.Y.) 39-46 (Hong Kong Association of Healthcare, Hong Kong, 1999).
- Liu, F. et al. Analysis of immunomodulating cytokines mRNAs in the mouse induced by mushroom polysaccharides. Life Sci.64, 1005-1011 (1999).
- Gu, Z.L. et al. Effects of Coriolus versicolor polysaccharopeptide on production of IL-6 from human peripheral blood lymphocytes. in Advanced research in PSP (ed. Yang, Q.Y.) 99-103 (Hong Kong Association of Healthcare, Hong Kong, 1999).
- Xu, L.Z. The antitumor and anti-virus activity of polysaccharopeptide (PSP). in Advanced research in PSP (ed. Yang, Q.Y.) 62-67 (Hong Kong Association of Healthcare, Hong Kong, 1999).
- Hsieh, T.C. et al. Effects of extracts of Coriolus versicolor (I'm Yunity) on cell-cycle progression and expression of Interleukins-1β, -6, and -8 in promyelocytic HL-60 leukemic cells and mitogenically stimulated and nonstimulated human lymphocytes. J. Altern. Complem. Med. 8, 591-602 (2002).
- Kevin, K.W. et al. Coriolus versicolor: A medicinal mushroom with promising immunotherapeutic values. J. Clin. Pharamcol.42, 976-984 (2002).
- Tsang, K.W. et al. Coriolus versicolor polysaccharide peptide slows progression of advanced non-small cell lung cancer. Resp. Med.97, 618-624 (2003).
- Kidd, P.M. The use of mushroom glucans and proteoglycans in cancer treatment. Altern. Med. Rev.5, 4-27 (2000).


